 Author
Author  Correspondence author
Correspondence author
Journal of Vaccine Research, 2024, Vol. 14, No. 3
Received: 08 Apr., 2024 Accepted: 12 May, 2024 Published: 24 May, 2024
This study comprehensively examines the development trajectory of mRNA vaccine technology, highlighting its historical evolution, fundamental mechanisms, technological advancements, and various applications. We trace key milestones in mRNA technology, including crucial innovations in mRNA stability, delivery systems, and large-scale manufacturing. The study discusses the successful application of mRNA vaccines in infectious diseases, particularly during the COVID-19 pandemic, and explores their prospects in cancer immunotherapy. Despite their transformative potential, mRNA vaccines still face challenges related to safety, efficacy, regulatory and ethical issues, and public acceptance. Future research should emphasize next-generation vaccines, integration with other technologies, and global health impacts. This study underscores the revolutionary impact of mRNA vaccines on modern medicine and public health.
1 Introduction
Vaccines have played a pivotal role in the advancement of public health, providing critical defense against infectious diseases and significantly reducing morbidity and mortality worldwide. From the pioneering work of Edward Jenner with the smallpox vaccine in the late 18th century to the development of modern vaccines using live-attenuated, inactivated, and subunit technologies, the field of vaccinology has continually evolved to address emerging health threats (Jackson et al., 2020). Traditional vaccines, which typically involve the introduction of an inactivated or attenuated pathogen, or parts of the pathogen, have been highly effective in preventing diseases such as polio, measles, and influenza. However, these conventional approaches often come with challenges, including lengthy development times, production complexities, and limitations in eliciting robust immune responses across diverse populations (Pardi et al., 2018).
In recent years, messenger RNA (mRNA) vaccines have emerged as a revolutionary platform in the field of vaccinology. Unlike traditional vaccines, mRNA vaccines work by introducing a synthetic mRNA sequence into the body, which encodes the antigen of interest. Host cells then translate this mRNA into protein, prompting an immune response that includes both humoral and cellular immunity. This approach not only mimics the natural infection process but also allows for rapid development and high scalability, making it a particularly attractive option for responding to pandemics and other emerging infectious diseases (Kim et al., 2021). The development of lipid nanoparticle (LNP) technology has further enhanced the stability and delivery of mRNA vaccines, ensuring efficient uptake and expression of the mRNA by host cells (Hassett et al., 2019).
The emergence of mRNA vaccine technology represents a paradigm shift in vaccine development, offering unprecedented speed, flexibility, and efficacy. This review explores the development trajectory of mRNA vaccine technology, detailing its historical evolution, fundamental mechanisms, technological advancements, and applications in infectious disease and cancer immunotherapy. By examining the challenges and controversies associated with mRNA vaccines, as well as future directions and potential impacts on global health, we aim to provide a comprehensive understanding of this transformative technology and its role in the future of vaccinology. This study underscores the importance of mRNA vaccines in addressing current and future public health challenges.
2 Historical Background of mRNA Vaccine Development
2.1 Early research and conceptualization
The concept of using messenger RNA (mRNA) for therapeutic purposes dates back several decades, with early research exploring its potential as a versatile and efficient platform for protein expression. Initial studies in the 1990s demonstrated that mRNA could be used to produce proteins in vitro and in vivo, sparking interest in its potential applications for vaccine development and gene therapy (Pardi et al., 2020). Researchers faced significant challenges, including the inherent instability of mRNA and its rapid degradation by nucleases, which limited its practical use. However, advancements in nucleotide modification and the development of more stable mRNA constructs helped overcome these initial hurdles (Gote et al., 2023).
2.2 Technological milestones
Several key technological milestones marked the evolution of mRNA vaccine development. Figure 1 clearly illustrates the notable progress in mRNA technology in both basic and applied research fields since 1961. These advancements have driven the development of mRNA vaccines and therapeutics, providing an essential historical context and reference for future research. One of the most significant advancements was the development of lipid nanoparticle (LNP) delivery systems. LNPs protect mRNA from degradation and facilitate its delivery into host cells, where it can be translated into the target protein (Hassett et al., 2019). Additionally, the introduction of modified nucleosides in mRNA sequences significantly improved the stability and translation efficiency of mRNA, reducing its immunogenicity and enhancing its therapeutic potential (Jackson et al., 2020).
.png) Figure 1 Timeline of Key Discoveries and Advancements in mRNA Technology (Adapted from Xu et al., 2020) Image caption: The green boxes represent discoveries and advancements in the mechanisms of mRNA, while the blue boxes indicate discoveries and advancements in the applications of mRNA drugs. Key technological advancements in mRNA mentioned in the figure include the 5' cap structure, lipid nanoparticles (LNP), and dendritic cells (DCs) research. The timeline also marks significant progress related to the COVID-19 pandemic, highlighting the potential of mRNA technology in addressing global infectious diseases (Adapted from Xu et al., 2020) |
Another critical milestone was the successful demonstration of mRNA vaccines in preclinical models. Studies showed that mRNA vaccines could induce strong immune responses and provide protection against various infectious diseases, laying the groundwork for further development (Zhang et al., 2019). These advancements culminated in the establishment of scalable manufacturing processes, enabling the rapid production of high-quality mRNA vaccines (Rosa et al., 2021).
2.3 Pre-COVID-19 applications
Before the COVID-19 pandemic, mRNA vaccines were already being investigated for a range of infectious diseases and therapeutic applications. Early clinical trials focused on viral infections such as influenza, rabies, and Zika, demonstrating the versatility and potential of mRNA vaccines (Pardi et al., 2018). Moreover, mRNA technology was explored for cancer immunotherapy, where it was used to encode tumor-associated antigens to stimulate an anti-tumor immune response (Maruggi et al., 2019). These pre-COVID-19 efforts provided critical insights and established a foundation for the rapid development and deployment of mRNA vaccines during the pandemic.
The development of mRNA vaccines before the COVID-19 era highlighted their potential to address a wide array of medical challenges, setting the stage for their pivotal role in the global response to the pandemic. The pre-existing research and technological infrastructure were instrumental in the swift creation and approval of mRNA-based COVID-19 vaccines, showcasing the readiness of this platform to tackle emergent health crises (Kim et al., 2021).
3 Mechanism of Action of mRNA Vaccines
3.1 Structure and composition of mRNA vaccines
mRNA vaccines are composed of a few critical components that ensure their stability and functionality. The primary element is the mRNA molecule itself, which encodes the antigen of interest. This mRNA is synthesized in vitro using a DNA template and includes modifications such as a 5' cap and a poly(A) tail to enhance its stability and translation efficiency (Gote et al., 2023). Additionally, the mRNA is often modified with pseudouridine or other nucleoside analogs to reduce its immunogenicity and increase its half-life within the host cells (Pardi et al., 2020).
The mRNA is encapsulated in lipid nanoparticles (LNPs), which serve multiple purposes: they protect the mRNA from degradation, facilitate its uptake by cells, and promote endosomal escape into the cytoplasm where translation occurs (Hassett et al., 2019). LNPs are typically composed of ionizable lipids, cholesterol, phospholipids, and polyethylene glycol (PEG)-lipid, which together form a stable and efficient delivery vehicle (Jackson et al., 2020).
3.2 Cellular mechanisms
The immune response generated by mRNA vaccines is typically strong and includes the activation of memory cells, which provide long-lasting immunity. This comprehensive immune activation mimics the body's natural response to infection, resulting in effective protection against the target pathogen (Iavarone et al., 2017). Figure 2 details the entire process of mRNA from transcription to immune activation.
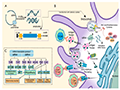 Figure 2 Process of In Vitro Transcription of mRNA and Innate Immune Activation (Adapted from Xu et al., 2020) Image caption: Figure 2A describes the in vitro transcription of mRNA using a DNA template containing an antigen-coding sequence, producing single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA) products; Figure 2B illustrates how mRNA enters the host cell cytoplasm through endocytosis. Some of the mRNA binds to host cell ribosomes and is successfully translated, producing antigen proteins. These proteins can be degraded into antigen peptides by proteasomes in the cytoplasm and presented to cytotoxic T lymphocytes (CTLs) via the major histocompatibility complex (MHC) class I pathway; Figure 2C explains the self-adjuvant effect of mRNA. The figure shows how various pattern recognition receptors (PRRs) can recognize mRNA in vitro transcription products, triggering the activation of antigen-presenting cells (APCs) and inflammatory responses (Adapted from Xu et al., 2020) |
Once administered, the LNP-encapsulated mRNA is taken up by host cells through endocytosis. Inside the cells, the LNPs facilitate the release of mRNA into the cytoplasm. Here, the mRNA is translated by the host cell's ribosomes into the target antigen, typically a viral protein, which then undergoes proper folding and post-translational modifications (Kim et al., 2021).
The expressed protein is processed and presented on the cell surface via major histocompatibility complex (MHC) molecules. This presentation triggers a robust immune response, including both humoral and cellular immunity. The antigen-presenting cells (APCs), such as dendritic cells, play a crucial role by migrating to the lymph nodes and activating T cells and B cells. T cells help orchestrate the immune response, while B cells differentiate into plasma cells that produce specific antibodies against the antigen (Pardi et al., 2018).
3.3 Delivery systems
The delivery system is a critical component of mRNA vaccines, with lipid nanoparticles (LNPs) being the most commonly used vehicle. LNPs are designed to encapsulate the mRNA and protect it from enzymatic degradation, ensuring its stability and enhancing cellular uptake. The composition of LNPs typically includes ionizable lipids, which facilitate endosomal escape, allowing the mRNA to reach the cytoplasm where translation occurs (Hassett et al., 2019).
Several advancements have been made in the optimization of LNP formulations to improve their efficacy and safety. For example, the use of biodegradable ionizable lipids has shown promise in reducing potential toxicity while maintaining high levels of protein expression and immunogenicity (Zeng et al., 2020). Additionally, the development of targeted delivery systems aims to direct the LNPs to specific cell types, enhancing the vaccine's effectiveness and reducing off-target effects (Buschmann et al., 2021).
Innovative delivery systems beyond LNPs are also being explored. These include polymer-based nanoparticles, peptides, and other nanomaterial-based carriers, each with unique properties that can potentially enhance the delivery and efficacy of mRNA vaccines. The choice of delivery system can significantly influence the vaccine's performance, including its ability to induce a strong and durable immune response (Liang et al., 2021).
4 Technological Advances in mRNA Vaccines
4.1 mRNA modifications and stability
One of the critical challenges in the development of mRNA vaccines has been ensuring the stability and longevity of the mRNA molecule in vivo. Early mRNA constructs were highly unstable and prone to rapid degradation by ribonucleases. To address this, researchers have developed several modifications to enhance mRNA stability and reduce immunogenicity. Incorporating modified nucleosides, such as pseudouridine and N1-methyl-pseudouridine, has been shown to significantly increase mRNA stability and translation efficiency while reducing the activation of innate immune sensors (Pardi et al., 2020).
The addition of a 5' cap and a poly(A) tail to the mRNA structure also plays a crucial role in enhancing its stability and translation. The 5' cap structure protects the mRNA from exonucleases and is recognized by the cellular machinery responsible for translation initiation, thereby increasing the efficiency of protein synthesis (Gote et al., 2023). Additionally, optimizing the codon usage to match the host cell's tRNA pool can further enhance the translation efficiency of the mRNA (Kim et al., 2021).
4.2 Delivery technologies
Effective delivery of mRNA into cells is a critical aspect of mRNA vaccine technology. Lipid nanoparticles (LNPs) have emerged as the most successful delivery vehicles, providing protection to the mRNA and facilitating its entry into cells. LNPs are typically composed of ionizable lipids, cholesterol, phospholipids, and polyethylene glycol (PEG)-lipid conjugates. These components work together to form stable particles that can encapsulate the mRNA, protect it from degradation, and promote cellular uptake (Hassett et al., 2019).
Recent advancements in LNP technology have focused on improving the efficiency and safety of these delivery systems. For example, the development of biodegradable ionizable lipids has reduced the potential toxicity associated with LNPs while maintaining high levels of protein expression and immunogenicity (Jackson et al., 2020). Additionally, targeted delivery systems are being explored to direct the LNPs to specific cell types or tissues, thereby enhancing the vaccine's effectiveness and minimizing off-target effects (Buschmann et al., 2021).
Beyond LNPs, other delivery technologies are also being investigated. These include polymer-based nanoparticles, peptides, and other nanomaterial-based carriers. Each of these delivery systems has unique properties that can potentially enhance the delivery and efficacy of mRNA vaccines. For instance, polymer-based nanoparticles can provide sustained release of mRNA, while peptide-based systems can facilitate targeted delivery to specific cell types (Liang et al., 2021).
4.3 Scalability and manufacturing
The scalability and manufacturing of mRNA vaccines have been major considerations, especially in the context of responding to global health emergencies such as the COVID-19 pandemic. One of the significant advantages of mRNA vaccine technology is its potential for rapid and scalable production. Unlike traditional vaccines, which often require the cultivation of live viruses or bacteria, mRNA vaccines can be produced using cell-free systems. This simplifies the production process and allows for rapid scaling (Rosa et al., 2021).
The manufacturing process typically involves the in vitro transcription of mRNA from a DNA template, followed by purification and encapsulation in LNPs. Advances in purification techniques, such as chromatography and tangential flow filtration, have improved the efficiency and yield of mRNA production. Furthermore, the modular nature of mRNA vaccine production allows for the rapid adaptation of the manufacturing process to produce vaccines against new or emerging pathogens (Jackson et al., 2020).
To meet the global demand for mRNA vaccines, companies have invested in expanding their production capacities and optimizing manufacturing workflows. Innovations in automation and process optimization have also contributed to the ability to produce large quantities of mRNA vaccines efficiently. These advancements have positioned mRNA vaccines as a promising platform for addressing not only current but also future public health challenges (Gote et al., 2023).
5 mRNA Vaccines in Infectious Diseases
5.1 Influenza and other viral infections
Before the advent of COVID-19, significant efforts were made to develop mRNA vaccines for a variety of viral infections, including influenza. Traditional influenza vaccines, which rely on inactivated or attenuated viruses, face challenges such as the need for annual reformulation and varying effectiveness. mRNA vaccines offer a promising alternative due to their rapid development cycle and the ability to encode multiple antigens, potentially enhancing their effectiveness (Pardi et al., 2018).
Studies on mRNA vaccines for influenza have demonstrated their ability to induce strong immune responses and provide protection in preclinical models. For instance, mRNA vaccines encoding hemagglutinin, a key influenza virus surface protein, have shown to be effective in eliciting neutralizing antibodies and T-cell responses, offering protection against influenza challenge in animal models (Jackson et al., 2020). Moreover, the flexibility of mRNA technology allows for the rapid adaptation of vaccines to match circulating strains, addressing the antigenic drift and shift that characterize influenza viruses.
Beyond influenza, mRNA vaccine platforms have also been explored for other viral infections such as rabies, Zika, and cytomegalovirus. These studies have consistently shown that mRNA vaccines can induce potent immune responses and provide protection against various viral pathogens, underscoring the broad applicability of this technology (Zhang et al., 2019).
5.2 COVID-19 pandemic response
The COVID-19 pandemic accelerated the development and deployment of mRNA vaccines, highlighting their potential in addressing global health crises. The rapid spread of SARS-CoV-2 and the urgent need for an effective vaccine led to the unprecedented speed at which mRNA vaccines were developed, tested, and authorized for emergency use. The Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) vaccines were the first mRNA vaccines to be widely used in humans, showcasing the ability of mRNA technology to respond quickly to emerging infectious diseases (Kim et al., 2021).
Arunachalam et al. (2021) comprehensively analyzed the innate and adaptive immune responses of 56 healthy volunteers following vaccination with the Pfizer-BioNTech mRNA vaccine (BNT162b2) (Figure 3). The study found that post-vaccination, volunteers generated neutralizing antibodies against both the SARS-CoV-2 prototype virus and the B.1.351 variant, with a significant increase in antigen-specific multifunctional CD4 and CD8 T cells after the second dose. The research showed that the second vaccine dose induced a stronger innate immune response compared to the first dose, including an increased frequency of CD14+CD16+ inflammatory monocytes, elevated plasma IFNγ concentrations, and transcriptional signatures of antiviral innate immunity. Additionally, single-cell transcriptomic analysis revealed a roughly 100-fold increase in myeloid cell populations enriched with interferon response transcription factors after the second immunization. The study also identified specific innate pathways associated with CD8 T cell and neutralizing antibody responses and demonstrated that monocyte-related features correlated with the neutralizing antibody response to the B.1.351 variant. Overall, these data reveal the immune responses induced by the mRNA vaccine and demonstrate its ability to elicit a stronger innate immune system response upon booster immunization.
 Figure 3 Changes in Antibody and T-Cell Responses Following BNT162b2 Vaccination Image caption: Figure 3a and Figure 3b show the binding antibody and neutralizing antibody responses after the first and second doses of the vaccine, respectively. The results indicate that most subjects generated an antibody response after the first dose, and the second dose significantly boosted this response; Figure 3c and Figure 3d display the responses of CD4+ and CD8+ T cells following vaccination. After the second dose, the frequency of antigen-specific CD4+ and CD8+ T cells significantly increased. These cells exhibited multifunctionality, including the simultaneous production of various cytokines |
Both vaccines demonstrated high efficacy in preventing COVID-19, with clinical trials showing efficacy rates of approximately 95% in preventing symptomatic infection. The rapid development was facilitated by several factors, including pre-existing mRNA technology platforms, advances in lipid nanoparticle delivery systems, and substantial financial and logistical support from governments and international organizations (Hassett et al., 2019).
The success of mRNA vaccines during the COVID-19 pandemic has also demonstrated their potential for rapid scalability and manufacturing. The modular nature of mRNA production allows for quick adaptation to new viral variants, which is crucial for maintaining vaccine efficacy as the virus evolves (Rosa et al., 2021). This experience has paved the way for future pandemic preparedness and the development of vaccines for other emerging infectious diseases.
5.3 Other infectious disease applications
Beyond influenza and COVID-19, mRNA vaccines have shown promise in addressing a range of other infectious diseases. For instance, mRNA vaccines targeting the Zika virus have been developed and tested in preclinical and early-phase clinical studies, demonstrating the ability to induce robust immune responses and provide protection against Zika infection (Pardi et al., 2020). Similarly, mRNA vaccines against rabies have shown efficacy in animal models, offering a potential new approach to preventing this deadly disease.
Research is also ongoing for mRNA vaccines targeting other pathogens such as cytomegalovirus (CMV), human immunodeficiency virus (HIV), and respiratory syncytial virus (RSV). These vaccines leverage the ability of mRNA technology to encode complex antigens and induce both humoral and cellular immune responses, which are essential for protection against these challenging pathogens (Liang et al., 2021).
The versatility of mRNA vaccines also extends to their potential use in combination vaccines, where multiple mRNA sequences encoding different antigens can be included in a single formulation. This approach could simplify vaccination schedules and improve compliance, particularly in regions with limited access to healthcare (Gote et al., 2023).
Overall, the success of mRNA vaccines in various infectious disease applications highlights their transformative potential in modern vaccinology. Continued research and development in this field are expected to yield new vaccines that can address both existing and emerging health threats, ultimately contributing to global health security.
6 mRNA Vaccines in Cancer Immunotherapy
6.1 Mechanisms and strategies
mRNA vaccines represent a promising approach in cancer immunotherapy, leveraging the body's immune system to target and eliminate cancer cells. The fundamental mechanism involves the delivery of mRNA encoding tumor-associated antigens (TAAs) into the patient's cells, which then produce the encoded antigens. These antigens are processed and presented on the surface of cells via major histocompatibility complex (MHC) molecules, triggering an immune response (Xu et al., 2023).
Several strategies have been employed to enhance the efficacy of mRNA cancer vaccines. These include the use of personalized mRNA vaccines, where neoantigens unique to the patient's tumor are identified and encoded into the mRNA. This personalized approach aims to create a highly specific immune response against the cancer cells while minimizing damage to normal tissues. Additionally, combining mRNA vaccines with other immunotherapies, such as checkpoint inhibitors, can further potentiate the anti-tumor immune response (Pardi et al., 2020).
6.2 Clinical trials and applications
Clinical trials have provided encouraging results for mRNA vaccines in cancer treatment. For instance, mRNA vaccines targeting melanoma have shown the ability to induce robust immune responses and achieve clinical benefits in patients. One notable example is the mRNA-4157 vaccine developed by Moderna, which is designed to encode multiple neoantigens specific to the patient's tumor. Clinical trials have demonstrated its safety and potential efficacy in combination with pembrolizumab, a PD-1 checkpoint inhibitor, in patients with advanced melanoma (Maruggi et al., 2019).
Other cancer types, such as non-small cell lung cancer (NSCLC) and colorectal cancer, are also being targeted by mRNA vaccines in clinical studies. These trials aim to evaluate the safety, immunogenicity, and therapeutic efficacy of mRNA vaccines either as monotherapies or in combination with other treatments. The results thus far have been promising, indicating that mRNA vaccines can induce specific anti-tumor immune responses and contribute to tumor regression (Zeng et al., 2020).
6.3 Future prospects
The future of mRNA vaccines in cancer immunotherapy looks promising, with several advancements on the horizon. One significant area of focus is the improvement of delivery systems to enhance the stability and targeting efficiency of mRNA vaccines. Innovations such as nanoparticle-based delivery systems and advanced lipid formulations are being explored to optimize the delivery of mRNA to the desired cells and tissues (Liang et al., 2021).
Additionally, the integration of mRNA vaccine technology with other therapeutic modalities, such as adoptive cell transfer and oncolytic viruses, holds the potential to create synergistic effects and improve treatment outcomes. The ability to rapidly design and produce mRNA vaccines also means they can be quickly adapted to target new and emerging cancer antigens, providing a versatile platform for cancer immunotherapy (Xu et al., 2023).
Furthermore, ongoing research aims to identify biomarkers that can predict patient response to mRNA vaccines, allowing for more personalized and effective treatment strategies. As the understanding of tumor immunology and mRNA technology continues to advance, it is likely that mRNA vaccines will play an increasingly important role in the fight against cancer, offering new hope for patients with various malignancies (Chen et al., 2022).
7 Challenges and Controversies
7.1 Safety and efficacy concerns
Despite the promising advancements in mRNA vaccine technology, safety and efficacy remain primary concerns that need continuous monitoring and evaluation. One of the main safety issues is the potential for acute adverse reactions, such as inflammation at the injection site, fever, and muscle pain. While these side effects are generally mild and transient, there have been rare cases of severe allergic reactions, such as anaphylaxis, particularly with the lipid nanoparticles (LNPs) used in mRNA vaccine delivery (Rosa et al., 2021).
Long-term safety data are still being collected, and it is essential to understand the implications of repeated mRNA vaccine doses and potential long-term effects on the immune system. There is also the concern about the durability of the immune response. While initial efficacy results have been promising, it remains to be seen how long the immunity lasts and whether booster doses will be required regularly (Gote et al., 2023).
Another concern is the theoretical risk of unintended immune responses, such as the development of autoimmunity, due to the immune system's response to the introduced mRNA or the encoded proteins. Continued vigilance and long-term studies are necessary to ensure that mRNA vaccines do not inadvertently trigger harmful immune responses (Pardi et al., 2020).
7.2 Regulatory and ethical considerations
The rapid development and deployment of mRNA vaccines, particularly during the COVID-19 pandemic, have raised several regulatory and ethical issues. Traditional vaccine development involves a lengthy process of clinical trials and regulatory review to ensure safety and efficacy. However, the urgent need for COVID-19 vaccines led to the use of emergency use authorizations (EUAs), which expedited the approval process but also sparked debates about the adequacy of the data supporting these decisions (Chen et al., 2022).
Ethical considerations also arise from the prioritization of vaccine distribution. Decisions about which populations receive the vaccine first, especially in the context of limited initial supply, involve ethical judgments about risk, equity, and fairness. These decisions must balance the need to protect vulnerable populations with the goal of achieving widespread immunity (Jackson et al., 2020).
Moreover, the global nature of the pandemic highlights disparities in vaccine access between high-income and low-income countries. Ensuring equitable distribution and addressing logistical challenges in diverse healthcare settings are crucial ethical imperatives. International cooperation and commitment to global health equity are necessary to ensure that mRNA vaccines reach all populations in need (Zeng et al., 2020).
7.3 Public acceptance and misinformation
Public acceptance of mRNA vaccines is a significant factor in the success of vaccination campaigns. Despite the scientific evidence supporting the safety and efficacy of mRNA vaccines, public hesitancy and resistance remain substantial challenges. Misinformation and conspiracy theories, often spread through social media, can undermine public trust in vaccines. False claims about vaccine ingredients, potential side effects, and the speed of vaccine development have fueled skepticism and reluctance to get vaccinated (Kim et al., 2021).
Addressing public concerns requires transparent communication from health authorities and scientists. Clear and consistent messaging about the safety, efficacy, and benefits of mRNA vaccines is essential. Public health campaigns must also engage with communities to understand their concerns and provide accurate information that can dispel myths and build trust (Gote et al., 2023).
Educational initiatives that explain the science behind mRNA vaccines and highlight the rigorous processes involved in their development can also help increase public confidence. Engaging with healthcare providers to equip them with the knowledge and tools to address patients' questions and concerns is another critical strategy in combating misinformation and enhancing vaccine uptake (Liang et al., 2021).
8 Future Directions and Perspectives
8.1 Next-Generation mRNA vaccines
The future of mRNA vaccine technology is poised to build on the successes and lessons learned from current mRNA vaccines. Next-generation mRNA vaccines aim to enhance the stability, efficacy, and delivery of mRNA constructs. Innovations such as self-amplifying mRNA (saRNA) are being explored, which allow for the mRNA to replicate within the host cell, potentially reducing the amount of mRNA required for vaccination and enhancing the immune response (Al Fayez et al., 2023).
Additionally, efforts are underway to improve the thermostability of mRNA vaccines, addressing the logistical challenges posed by the need for cold chain storage. Advances in lyophilization and other stabilization techniques could enable mRNA vaccines to be stored at higher temperatures, facilitating distribution in low-resource settings (Pardi et al., 2020). Furthermore, research into multi-epitope mRNA vaccines, which encode multiple antigens from different pathogens, could lead to the development of broad-spectrum vaccines capable of protecting against various diseases with a single injection (Gote et al., 2023).
8.2 Integration with other technologies
The integration of mRNA vaccine technology with other advanced biomedical technologies holds significant promise for enhancing vaccine efficacy and expanding their applications. For instance, combining mRNA vaccines with nanotechnology can improve targeted delivery and uptake by specific cells, increasing the precision and potency of the immune response (Zeng et al., 2020). Nanoparticle-based delivery systems can be engineered to enhance cellular uptake and reduce off-target effects, making mRNA vaccines more efficient and safer.
Artificial intelligence (AI) and machine learning (ML) are also being leveraged to accelerate mRNA vaccine development. These technologies can analyze vast amounts of data to identify optimal mRNA sequences and predict the best antigen targets, significantly speeding up the vaccine design process. AI and ML can also help optimize manufacturing processes, ensuring high yield and quality of mRNA vaccines (Liang et al., 2021).
Furthermore, integrating mRNA vaccines with other immunotherapeutic approaches, such as checkpoint inhibitors or adoptive cell therapies, can create synergistic effects that enhance anti-tumor and anti-viral responses. These combination therapies could revolutionize the treatment of cancers and chronic viral infections, offering new hope for patients who do not respond to conventional treatments (Jackson et al., 2020).
8.3 Global health implications
The global health implications of mRNA vaccine technology are profound, particularly in addressing infectious diseases and improving vaccine accessibility in low- and middle-income countries (LMICs). The rapid development and deployment of mRNA vaccines during the COVID-19 pandemic demonstrated their potential to respond swiftly to emerging health threats. However, ensuring equitable access to these vaccines remains a critical challenge (Rosa et al., 2021).
Efforts to expand manufacturing capacity and reduce costs are essential for making mRNA vaccines more accessible globally. Investments in regional production facilities and technology transfer agreements can help LMICs develop their own manufacturing capabilities, reducing dependency on imports and ensuring a more reliable supply of vaccines (Chen et al., 2022).
Moreover, mRNA vaccines offer the potential to address a wide range of infectious diseases that disproportionately affect LMICs, such as malaria, tuberculosis, and HIV. By targeting these diseases, mRNA vaccines could significantly reduce the global burden of infectious diseases and improve public health outcomes (Tan et al., 2023).
In conclusion, the evolution of mRNA vaccine technology holds immense promise for the future of global health. Continued innovation and investment in this field are essential to fully realize the potential of mRNA vaccines in preventing and treating a wide array of diseases, ultimately contributing to a healthier and more resilient global population (Gote et al., 2023).
9 Concluding Remarks
The evolution of mRNA vaccine technology represents a significant milestone in the field of vaccinology, offering new paradigms for vaccine development and disease prevention. The historical research and technological milestones of mRNA vaccines paved the way for their development, overcoming early challenges related to instability and delivery. The unique mechanism of action of mRNA vaccines involves the delivery of mRNA encoding specific antigens via lipid nanoparticles, triggering a robust immune response by mimicking the natural infection process.
Technological advancements have significantly enhanced the stability, efficacy, and accessibility of mRNA vaccines through innovations in mRNA modifications, delivery technologies, and scalable manufacturing processes. mRNA vaccines have demonstrated efficacy against a range of viral infections, including influenza, Zika, and notably, COVID-19, showcasing their rapid development potential and high efficacy.
In the realm of cancer immunotherapy, mRNA vaccines are being explored for various malignancies, showing promising results in clinical trials by encoding tumor-associated antigens and inducing specific immune responses. However, despite their success, mRNA vaccines face challenges related to safety, long-term efficacy, regulatory hurdles, and public acceptance, which underscore the need for ongoing vigilance and transparent communication.
The future of mRNA vaccines looks promising, with ongoing advancements aimed at improving vaccine stability, delivery, and integration with other technologies, as well as addressing global health disparities.
The rapid development and deployment of mRNA vaccines during the COVID-19 pandemic have undeniably revolutionized the field of vaccine technology. This unprecedented achievement has not only provided a critical tool in combating the pandemic but has also demonstrated the potential of mRNA vaccines to address a wide array of infectious diseases and cancer. The flexibility, speed, and efficacy of mRNA vaccines mark a new era in vaccinology, promising faster responses to future pandemics and personalized approaches to cancer treatment.
Looking ahead, the continued evolution of mRNA vaccine technology will depend on addressing the remaining challenges and leveraging technological advancements. Ensuring global equity in vaccine access, improving public trust through transparency, and enhancing vaccine formulations for broader protection are crucial steps towards maximizing the impact of mRNA vaccines on global health.
mRNA vaccines have set a new standard in the field of vaccinology, demonstrating remarkable potential to transform the prevention and treatment of diseases. As research and innovation continue, mRNA vaccines are poised to play a pivotal role in future public health strategies, offering hope for a healthier and more resilient global population.
Acknowledgments
The author expresses gratitude to the two anonymous peer reviewers for their feedback.
Conflict of Interest Disclosure
The author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Al Fayez N., Nassar M.S., Alshehri A.A., Alnefaie M.K., Almughem F.A., Alshehri B.Y., Alawad A.O., and Tawfik E.A., 2023, Recent advancement in mRNA vaccine development and applications, Pharmaceutics, 15(7): 1972.
https://doi.org/10.3390/pharmaceutics15071972
Arunachalam P.S., Scott M.K.D., Hagan T., Li C.F., Feng Y.P., Wimmers F., Grigoryan L., Trisal M., Edara V.V., Lai L.L., Chang S.E., Feng A., Dhingra S., Shah M., Lee A.S., Chinthrajah S., Sindher S.B., Mallajosyula V., Gao F., Sigal N., Kowli S., Gupta S., Pellegrini K., Tharp G., Maysel-Auslender S., Hamilton S., Aoued H., Hrusovsky K., Roskey M., Bosinger S.E., Maecker H.T., Boyd S.D., Davis M.M., Utz P.J., Suthar M.S., Khatri P., Nadeau K.C., and Pulendran B., 2021, Systems vaccinology of the BNT162b2 mRNA vaccine in humans, Nature, 596(7872): 410-416.
https://doi.org/10.1038/s41586-021-03791-x
Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., and Weissman D., 2021, Nanomaterial delivery systems for mRNA vaccines, Vaccines, 9(1): 65.
https://doi.org/10.3390/vaccines9010065
Chen J., Chen J., and Xu Q., 2022, Current developments and challenges of mRNA vaccines, Annual Review of Biomedical Engineering, 24(1): 85-109.
https://doi.org/10.1146/annurev-bioeng-110220-031722
Gote V., Bolla P.K., Kommineni N., Butreddy A., Nukala P.K., Palakurthi S.S., and Khan W., 2023, A comprehensive review of mRNA vaccines, International Journal of Molecular Sciences, 24(3): 2700.
https://doi.org/10.3390/ijms24032700
Hassett K., Benenato K., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B., Ketova T., Mihai C., Lynn A., McFadyen I., Moore M., Senn J., Stanton M., Almarsson Ö., Ciaramella G., and Brito L.A., 2019, Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines, Molecular Therapy-Nucleic Acids, 15: 1-11.
https://doi.org/10.1016/j.omtn.2019.01.013
Iavarone C., O'hagan D.T., Yu D., Delahaye N.F., and Ulmer J.B., 2017, Mechanism of action of mRNA-based vaccines, Expert Review of Vaccines, 16(9): 871-881.
https://doi.org/10.1080/14760584.2017.1355245
Jackson N.A., Kester K.E., Casimiro D., Gurunathan S., and DeRosa F., 2020, The promise of mRNA vaccines: a biotech and industrial perspective, npj Vaccines, 5(1): 11.
https://doi.org/10.1038/s41541-020-0159-8
Kim J., Eygeris Y., Gupta M., and Sahay G., 2021, Self-assembled mRNA vaccines, Advanced drug Delivery Reviews, 170: 83-112.
https://doi.org/10.1016/j.addr.2020.12.014
Liang Y., Huang L., and Liu T., 2021, Development and delivery systems of mRNA vaccines, Frontiers in Bioengineering and Biotechnology, 9: 718753.
https://doi.org/10.3389/fbioe.2021.718753
Maruggi G., Zhang C., Li J., Ulmer J.B., and Yu D., 2019, mRNA as a transformative technology for vaccine development to control infectious diseases, Molecular Therapy, 27(4): 757-772.
https://doi.org/10.1016/j.ymthe.2019.01.020
Pardi N., Hogan M.J., and Weissman D., 2020, Recent advances in mRNA vaccine technology, Current Opinion in Immunology, 65: 14-20.
https://doi.org/10.1016/j.coi.2020.01.008
Pardi N., Hogan M.J., Porter F.W., and Weissman D., 2018, mRNA vaccines-a new era in vaccinology, Nature reviews Drug discovery, 17(4): 261-279.
https://doi.org/10.1038/nrd.2017.243
Rosa S.S., Prazeres D.M., Azevedo A.M., and Marques M.P., 2021, mRNA vaccines manufacturing: Challenges and bottlenecks, Vaccine, 39(16): 2190-2200.
https://doi.org/10.1016/j.vaccine.2021.03.038
Tan T., Deng S.T., Wu B.H., Yang Q., Wu M.W., Wu H., Cao C.H., and Xu C., 2023, mRNA vaccine-A new cancer treatment strategy, Current Cancer Drug Targets, 23(9): 669-681.
https://doi.org/10.2174/1568009623666230222124424
Xu S., Yang K., Li R., and Zhang L., 2020, mRNA vaccine era-mechanisms, drug platform and clinical prospection, International Journal of Molecular Sciences, 21(18): 6582.
https://doi.org/10.3390/ijms21186582
Zeng C., Zhang C., Walker P.G., and Dong Y., 2020, Formulation and delivery technologies for mRNA vaccines, In Dong Y., and Benjamin P., eds., mRNA Vaccines, Springer International Publishing, Switzerland, pp.71-110.
https://doi.org/10.1007/82_2020_217
Zhang C., Maruggi G., Shan H., and Li J., 2019, Advances in mRNA vaccines for infectious diseases, Frontiers in Immunology, 10: 594.
https://doi.org/10.3389/fimmu.2019.00594

. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Xiaojie Zhang
Related articles
. mRNA vaccines
. Vaccine development
. Immune response
. Cancer immunotherapy
. COVID-19
Tools
. Post a comment